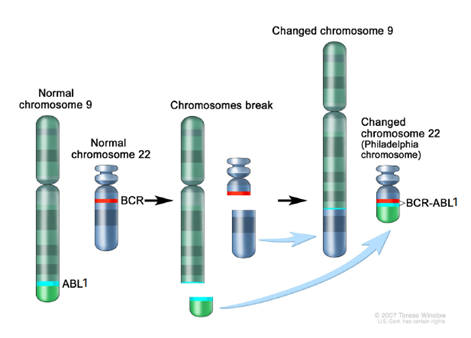
Dr. PCR™ BCR-ABL1 Major IS Detection Kit is an in vitro diagnostic solution designed for monitoring patients with Chronic Myeloid Leukemia (CML). The kit is designed for the quantification of BCR-ABL1 (e13a2 and e14a2) and ABL1 transcripts from RNA extracted from whole blood.
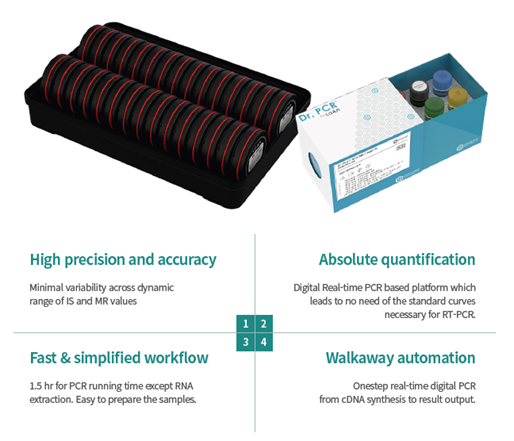
This is a digital PCR technology that stands out for its simplicity, speed, and degree of automation, including reverse transcription and amplification in a single step within the cartridge, and eliminating the need for calibration curves and replicates. All this reduces manual labor time to a minimum (10-15 min).
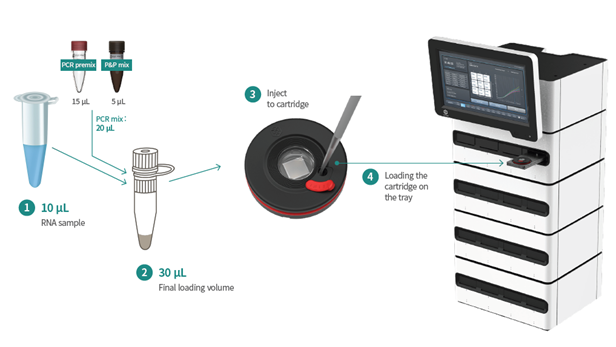
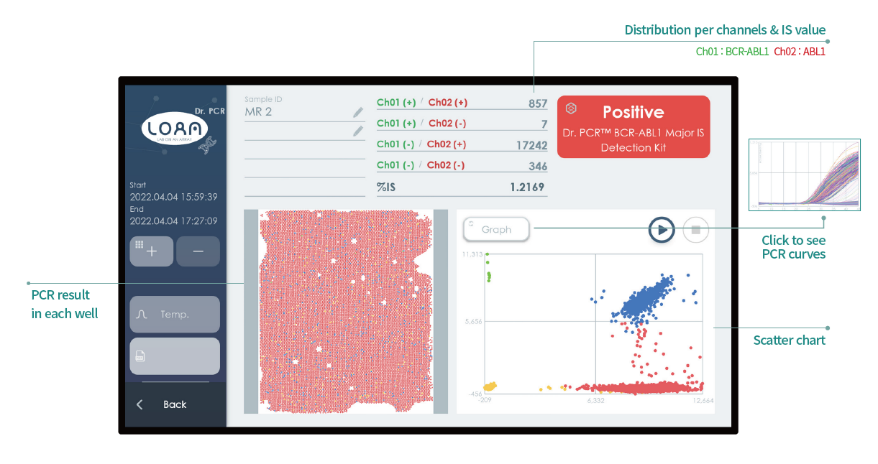
The dedicated digital PCR equipment features a simple interface and automatically calculates the result in %IS and molecular response (MR) in addition to reporting the absolute number of copies of both targets (BCR-ABL1 and ABL1). Two different devices are available, both desktop and compact in size:
- LOAA OnPoint: suitable for small sample numbers (works with 1 sample at a time)
- LOAA-M: suitable for larger numbers of samples and adaptable. It is a modular equipment that allows processing from 1 to 16 samples in parallel (4 per module) with continuous loading.
The performance of this solution is excellent, with high sensitivity and precision thanks to the real-time digital PCR technology developed by Optolane. With this approach, a final time reading of each partition is obtained, as well as a real-time reading of it, which allows the elimination of false positives by increasing the precision in targets that are found in low quantities.
Key aspects:
- Automated, fast, and simple solution: 1.5 h – 10 min of manual labor
- Starting sample: RNA extracted from peripheral blood (10 ul, 60-150 ng/ul)
- Includes reverse transcription and dPCR in one step
- Automatic calculation of MR, %IS, absolute number of copies
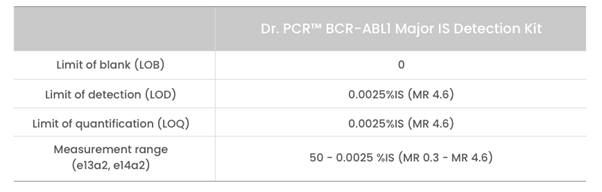
- High sensitivity and precision – LoD: MR 4.6 (0.0025% IS) / Measurement range: 50-0.0025 %IS
- CE-IVD marking à IVDR in progress
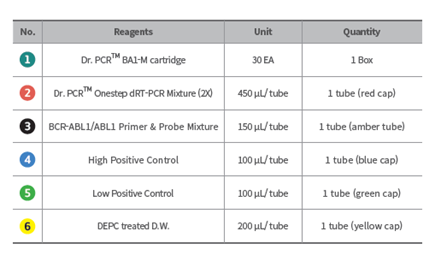
Components of the kit: