SCOPE™ Kits


KKit for the determination of the CTG triplet repeats number of the DMPK gene by fluorescent fragment analysis
Information about the product
Myotonic dystrophy type 1 or Steinert’s disease is currently the most common form of muscular dystrophy in adults. Inheritance of this multisystem disease is autosomal dominant, and phenotypic expression is highly variable due to an unstable expansion CTG trinucleotide repeats dystrophia myotonica protein kinase gene (DMPK, MIM*605377).
There is a correlation between the number of CTG repeats and the age of appearance and severity of symptoms:
Intended use
Adellgene® Myotonic Dystrophy Confirmatory is a semi-automated in vitro diagnostic kit designed for use in clinical laboratories which quantitatively determines the number of repetitions of CTG (cytosine-thymine-guanine) of 3´UTR region of the DMPK gene located in chromosome 19 resulting in Myotonic Dystrophy Type 1 (DM1) disease. It aims to aid diagnosis associated with clinical findings in DM1 that span from mild to severe symptoms.
The use of this kit is the confirmation of homozygous and detection of false homozygous for the occurrence of a higher range allele obtainable with Adellgene® Myotonic Dystrophy Screening kit.
The technology is based on the triplet repeat primed polymerase chain reaction (TP-PCR) of genomic DNA extracted from peripheral blood followed by fluorescence analysis of the PCR fragments obtained in a genetic analyzer.
Patients who can benefit from this determination are those referred by a specialist. The intended user of the kit is technical personnel trained to carry out the protocol and the interpretation of results described in the instructions for use.
Workflow

Results
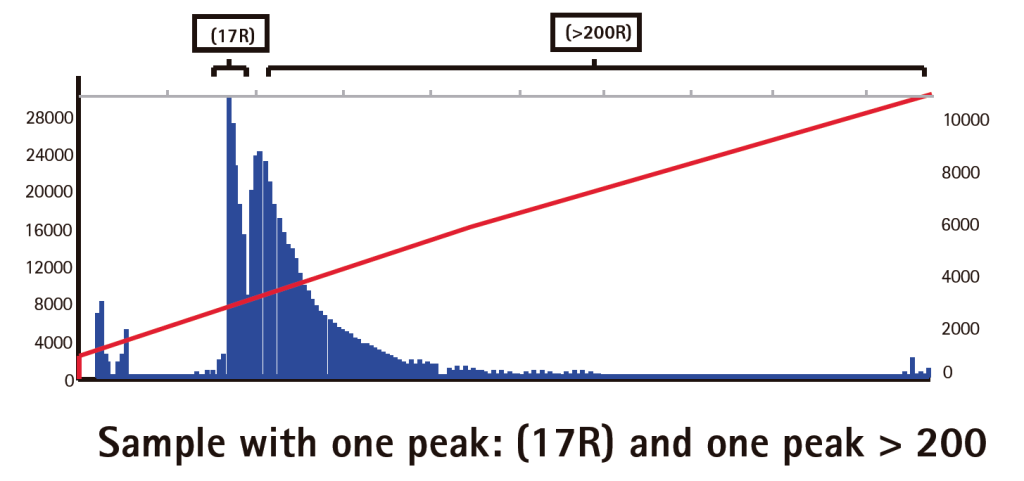
Limitations
| PRODUCTO | CANTIDAD |
|---|---|
| AD-MD-C-16 | 16 test/kit |
Public Documentation
Related products
2021 © Diagnóstica Longwood SL
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!
Más información sobre nuestra política de cookies
