SCOPE™ Kits


Kit for the determination of healthy and premutation alleles in the FMR1 gene by fluorescent fragment analysis
Information about the product
Fragile X Syndrome (FXS, OMIM # 300624) is an X-linked disease that is primarily based on the genomic expansion of a triplet of nucleotides (CGG), and aberrant methylation of the promoter region.
FXS has a prevalence of 1 in 4000 males and 1 in 8000 females. Affected individuals show a striking phenotype consisting on large ears and a prominent jaw.
Depending on the number of repetitions of this triplet, three categories can be established:
Intended use
Adellgene® Fragile X Screening is a semi-automated in vitro diagnostic kit designed for use in clinical laboratories, which quantitatively determines the number of CGG triplet repeats (cytosine-guanine-guanine) in the 5’ untranslated region of the gene for fragile X mental retardation (“Fragile X mental retardation-1”: FMR1). It aims to aid diagnosis of the clinical disease associated with Fragile X syndrome, for example, mental retardation, primary ovarian failure, and tremors / ataxia.
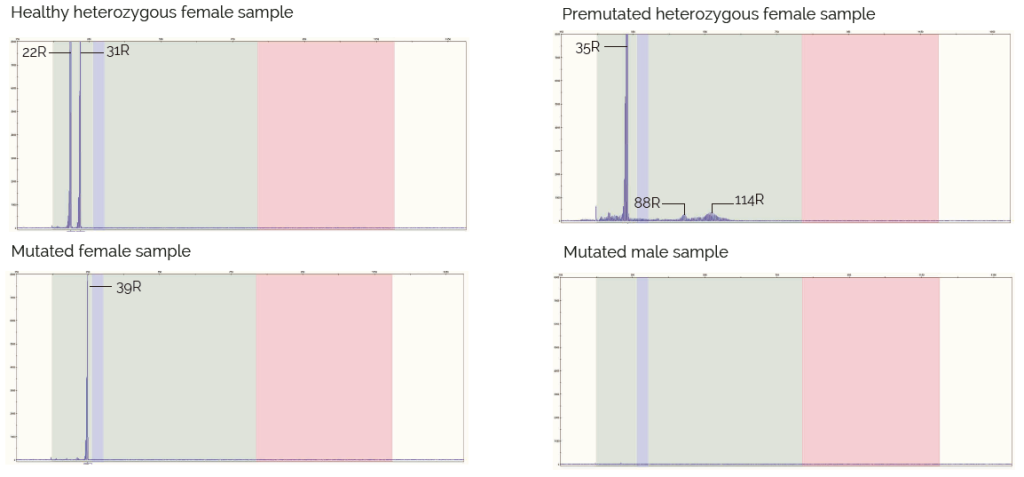
This kit can be used for the determination of the number of repeats present in healthy individuals (up to 45/55 CGG repeats) and premutated individuals (from 45/55 to 200 CGG triplet repeats). Female samples reporting only one peak in the results’ electropherogram and male samples with no peak must be analyzed using other appropriate technique, for example, TP-PCR, in order to verify the non-existence of a fully mutated allele (>200 CGG repeats).
The technology is based on the polymerase chain reaction (PCR) amplification of genomic DNA extracted from peripheral blood, followed by fluorescence analysis of the size of the PCR fragments obtained by a genetic analyzer and conversion of that size into the respective number of CGG repeats.
Patients who can benefit from this determination are those referred by a specialist. The intended user of the kit is technical personnel trained to carry out the protocol and the interpretation of results described in the Instructions for Use.
Workflow

Results
Limitations
| Presentación |
|---|
| 48 test/kit |
Public Documentation
Related products
2021 © Diagnóstica Longwood SL
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!
Más información sobre nuestra política de cookies
