dRAST™ allows rapid and direct antibiotic sensitivity testing from positive blood cultures, enabling therapy to be optimised for the critical treatment of sepsis.
- Direct: Phenotypic assessment of antimicrobial susceptibility directly from a positive blood culture.
- Rapid: Results for 12 samples in only 4-6 hours, saving up to 48 hours compared to conventional antibiograms.
- Simple: Intuitive and easy to use interface.
- Comprehensive solution: Antibiogram, analysis and interpretation of results (MIC and SIR classification).
- Integrated analysis software “dRAST Expert System”, which allows the incorporation of international guidelines and recommendations (EUCAST, CLSI and CA-SFM).

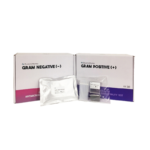
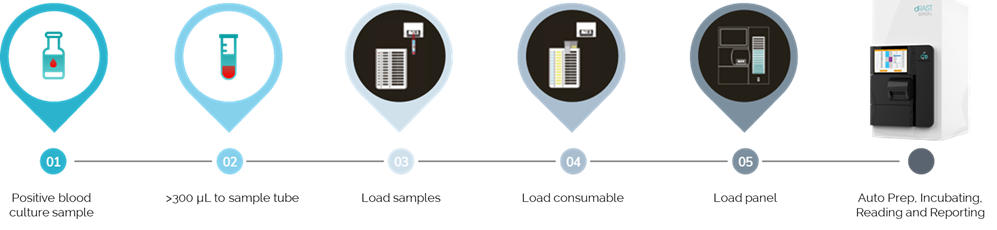
After a simple Gram stain, positive blood culture samples are loaded into the dRAST™ platform without any additional preparation, along with consumables and antibiotic panels for Gram-negative or Gram-positive bacteria, depending on the results of the initial staining.
dRAST™ combines 2 reference methods (broth microdilution and drug diffusion), with a patented system for time-lapse microscopic imaging.