Kit for detecting the G20210A mutation of the prothrombin gene by Real Time PCR using TaqMan® probes technology
Information about the product
Prothrombin (Factor II) is a glycoprotein generated in liver. It is an essential component of the blood-clotting mechanism. The regulation of the prothrombin expression is crucial for the homeostasis maintenance.
The G20210A mutation of Factor II presents a high prevalence (18%) in families with thrombosis and in 6.2% of patients with first thrombosis. The G20210A position within the prothrombin gene is explained to cause an increase in the level of prothrombin, which leads to an increased risk of thrombosis.
Intended Use
Genvinset® Factor II G20210A is a semi-automated kit for the in vitro qualitative detection of the G20210A mutation (NCBI dbSNP rs1799963; NM_000506.5:c.*97G>A) in the prothrombin (FII) gene (OMIM: 176930) associated with thrombophilia risk, in genomic DNA extracted from whole blood using Real Time PCR technology with specific TaqMan® probes.
Patients who can benefit from this determination are those referred by a specialist. The results of this test should not be the only ones on which the therapeutic decision is based and should be used as an aid in the diagnosis together with results of other markers of the disease.
The intended user of the kit is technical personnel trained to carry out the protocol and the interpretation of results described in the instructions for use.
Workflow

Results
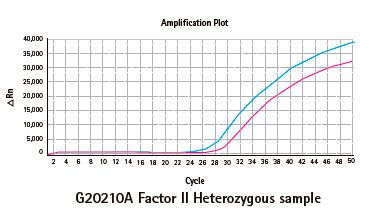
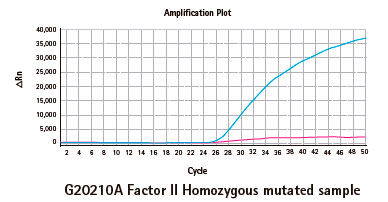
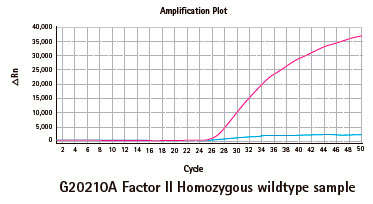

Limitations
- Mutations or polymorphisms at annealing primer/probe sites are possible and may result in the lack of allele definition. Other technologies could be necessary to resolve the typing.
- Data and result interpretation should be revised by qualified personnel.
- This product is an auxiliary tool for the diagnosis of patients with suspected thrombophilia. Use these results in conjunction with clinical data and results of other tests performed on the patient.