Kit for detecting the HLA-B*57:01 alleles by Real-Time PCR using TaqMan® probes technology
Information about the product
Abacavir sulfate is a synthetic carboxylic nucleoside drug, which works as a reverse transcriptase inhibitor and is used to treat HIV (Human Immunodeficiency Virus). This drug has been associated with the occurrence of fatal hypersensitivity reaction with symptoms as fever, skin rash, fatigue, nausea, vomiting or diarrhea.
The aforementioned hypersensitivity has been related to the HLA-B*57:01 allele so, before starting the abacavir treatment, it is recommended to perform a study to determine the presence or absence of the allele in the patient.
The HLA-B*57:01 allele is a polymorphism of the HLA-B gene, which belongs to the MHC (Major Histocompatibility Complex) class I. This allele shows a frequency of around 3% in the European population.
HLA-B*57:01 screening reduces the risk of hypersensitivity reaction to abacavir.
Intended Use
Genvinset® HLA B57v5 is a semi-automated in vitro diagnostic kit for the qualitative detection of the HLA-B*57:01 allele in genomic DNA extracted from whole blood, associated with abacavir hypersensitivity reaction, by Real-Time PCR using TaqMan® probes technology.
Patients who can benefit from this determination are those referred by a specialist. The results of this test can assess the administration of the convenient treatment and, in fact, HLA-B*57:01 analysis should be performed previous to the delivery of abacavir.
The intended user of the kit is technical personnel trained to carry out the protocol and the interpretation of results described in the Instructions for Use.
Workflow

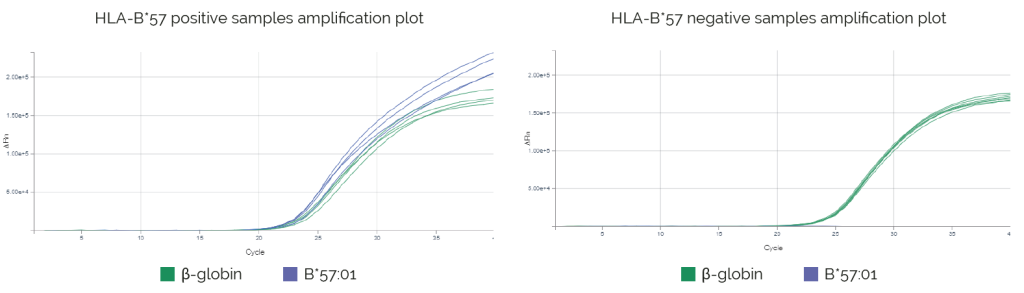
Results
Limitations
- Mutations or polymorphisms at annealing primer/probe sites are possible and may result in the lack of allele definition. Other technologies could be necessary to resolve the typing.
- Data and result interpretation should be revised by qualified personnel.
- The results of this test can help to assess the administration of the correct treatment, and in fact, the analysis of HLA-B*57:01 should be carried out before abacavir is given.