SCOPE™ Kits

CatalogImmunology and transplantationHLA-associated diseases

Insulin-dependent or type 1 diabetes mellitus (IDDM) is an autoimmune disorder characterized by destruction of insulin producing beta cells in the pancreas. Progression to disease involves both genetic and environmental factors.It is the most common form of diabetes among children and young adults in populations of Caucasoid origin, where the prevalence is approximately 0.4%. In the 1970s, association and affected-sib pair linkage studies established the role of HLA genes in IDDM predisposition.
Initial association studies of serologically determined HLA alleles with IDDM showed increased frequencies of B8-DR3 and B15 (w62)-DR4 in Caucasians. Both DR3 and DR4 are very strongly associated with IDDM.
It is clear that some combinations of HLA-DQ genes are associated with susceptibility to IDDM. From both human genetics and animal model studies there is good evidence that particular alleles of the HLA-DQB1 (*03:02 and *02:01) and DRB1 (*03 and *04) loci all are primarily involved in the genetic predisposition to IDDM.
Genvinset® HLA Diabetes Mellitus Type I is a semi-automated in vitro diagnostic kit for HLA-DRB1*03/ DRB1*04/ DQB1*02:01/ DQB1*03:02 alleles qualitative detection in genomic DNA extracted from whole blood, associated with diabetes mellitus type 1 predisposition, by Real Time PCR using TaqMan® probes technology.
Patients who can benefit from this determination are those referred by a specialist. The results of this test should not be the only ones on which the therapeutic decision is based and should be used as an aid in the diagnosis together with results of other markers of the disease.
The intended user of the kit is technical personnel trained to carry out the protocol and the interpretation of results described in the instructions for use.

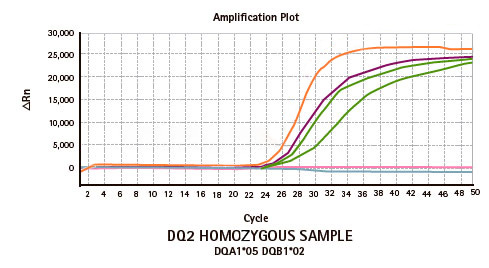
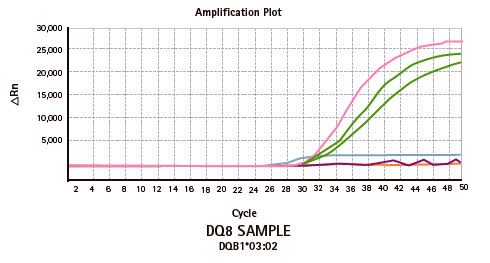
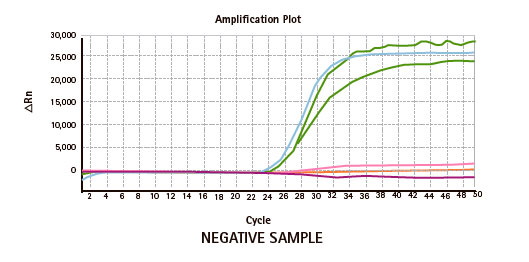
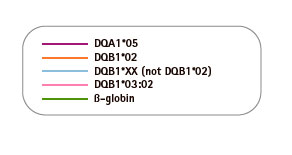
| PRODUCTO | CANTIDAD |
|---|---|
| GVS-DMT1-48 | 16 test/kit |
Public Documentation
Related products
2021 © Diagnóstica Longwood SL
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!
Más información sobre nuestra política de cookies
