SCOPE™ Kits

CatalogHematology and blood bankThrombophilias and Hemochromatosis

Kit for detecting the C677T polymorphism of the MTHFR gene by Real Time PCR using TaqMan® probes technology
Information about the product
Methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in folate metabolism. Although MTHFR protein does not participate in the clotting cascade as other proteins such as FII and FV (and therefore belonging to the well-known group of coagulation factors), the involvement of MTHFR in the folate metabolic pathway may affect thrombophilia development, as well as increased cancer risk or Alzheimer’s disease appearance.
MTHFR gen can present different polymorphisms. The most common and studied variant is the MTHFR C677T polymorphism but, also, the MTHFR A1298C mutation has been studied. The frequency of both MTHFR polymorphisms differs among the different ethnicities.
Intended use
Genvinset® MTHFR C677T is a semi-automated kit for the in vitro qualitative detection of the C677T polymorphism (NCBI dbSNP rs1801133; NM_001330358.2:c.788C>T) in the Methylene tetrahydrofolate reductase (MTHFR) gene (OMIM: 607093) associated with thrombophilia risk in genomic DNA extracted from whole blood using Real Time PCR technology with specific TaqMan® probes.
Patients who can benefit from this determination are those referred by a specialist. The results of this test should not be the only ones on which the therapeutic decision is based and should be used as an aid in the diagnosis together with results of other markers of the disease.
The intended user of the kit is technical personnel trained to carry out the protocol and the interpretation of results described in the instructions for use.
Workflow

Results
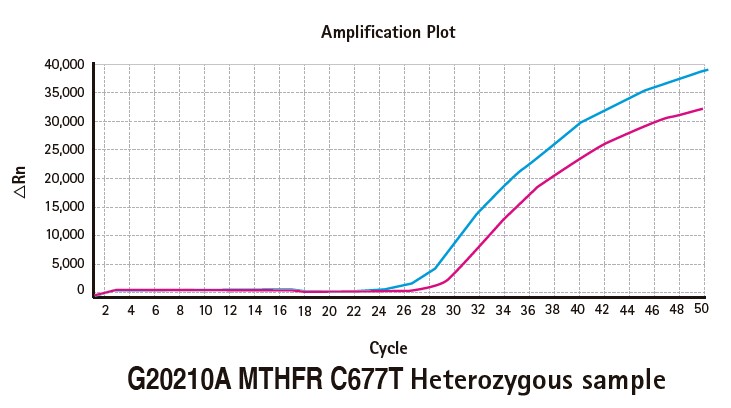
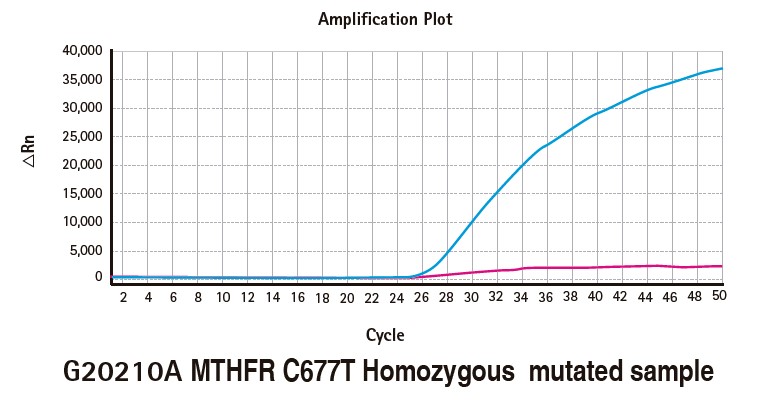
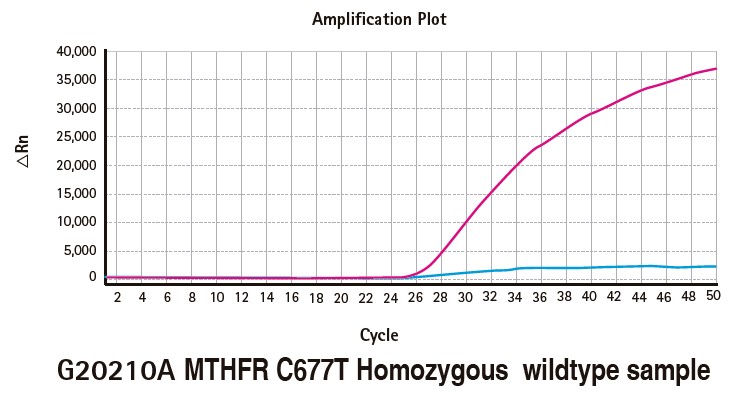

Limitations
| PRODUCT | PRESENTATION |
|---|---|
| GVS-MTHFR-48 | 48 test/kit |
Public Documentation
Related products
2021 © Diagnóstica Longwood SL
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
This website uses Google Analytics to collect anonymous information such as the number of visitors to the site, and the most popular pages.
Keeping this cookie enabled helps us to improve our website.
Please enable Strictly Necessary Cookies first so that we can save your preferences!
Más información sobre nuestra política de cookies
