Kit for detecting the C13910T and G22018A polymorphisms of the MCM6 gene by Real Time PCR using specific TaqMan® probes technology.
Information about the product
In most human beings, the ability to digest lactose rapidly decreases after the breastfeeding period (primary lactose intolerance). This is due to a reduction in the lactase-phlorizin hydrolase enzyme (LPH), which is responsible for the hydrolysis of lactose to glucose and galactose, sugars which are both easily absorbed in the intestine. However, some individuals maintain the ability to digest lactose as adults.
The frequency of persistence of lactase activity is high in north-European populations (>90% in Sweden and Denmark), whereas it progressively decreases towards the south of Europe and the Middle East (around 50% in France, Spain and some Arabic populations), and is very low in Asian and African populations.
Lactose intolerance causes several symptoms that include abdominal pain, bloating, diarrhoea and gas as the most common, and other wide-ranging symptoms such as nausea, headache, lack of concentration, severe fatigue, muscular and joint pains, etc.
Intended Use
Genvinset® Lactose Intolerance is a semi-automated in vitro diagnostic kit for the qualitative detection of the polymorphisms -13910 C/T (NCBI dbSNP rs4988235; NM_005915.6:c.1917+326C>T) and -22018 G/A (NCBI dbSNP rs182549; NM_005915.5:c.1362+117G>A) of the MCM6 gene (OMIM: 601806) associated with lactase persistence, in genomic DNA extracted from whole blood, by Real-Time PCR using specific TaqMan® probes technology.
Patients who can benefit from this determination are those referred by a specialist. The results of this test should not be the only ones on which the therapeutic decision is based and should be used as an aid in the diagnosis together with results of other markers of the disease.
The intended user of the kit is technical personnel trained to carry out the protocol and the interpretation of results described in the Instructions for use.
Workflow

Results
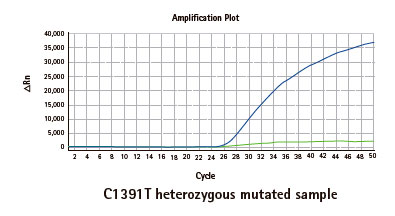
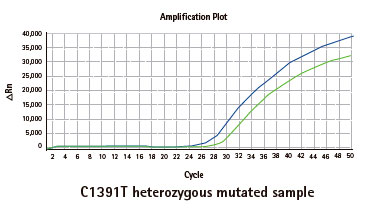
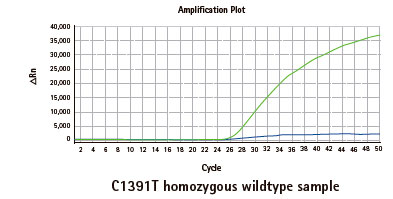

Limitations
- Mutations or polymorphisms at annealing primer/probe sites are possible and may result in the lack of allele definition. Other technologies could be necessary to resolve the typing.
- Data and result interpretation should be revised by qualified personnel.
- This product is an auxiliary tool for the diagnosis of patients with suspected lactose intolerance. Use these results in conjunction with clinical data and results of other tests performed on the patient.