The detection of antibodies against blood group antigens is crucial in pre- and post-transfusion test. There are cases in which the existing antibodies made difficult to identify them due to panagglutination reactions.
rBGA are recombinant proteins, equivalent to the the different blood groups antigens.
Pretreatment of the patient’s serum with rBGA (recombinant blood group antigens) allows a more precise detection of antibodies against the erythrocyte antigens, by specifically inhibiting the selected ones, minimizing the risk of incompatible blood transfusions.
- Specific blocking of antibodies against high frequency antigens for cross-transfusion pre-test.
- Detection and identification of antibodies in a single step.
- False positive reduction.
- Easy to implement in routine serology
The kits are compatible with commonly used commercial panels, allowing their use without modifying routine laboratory protocols.
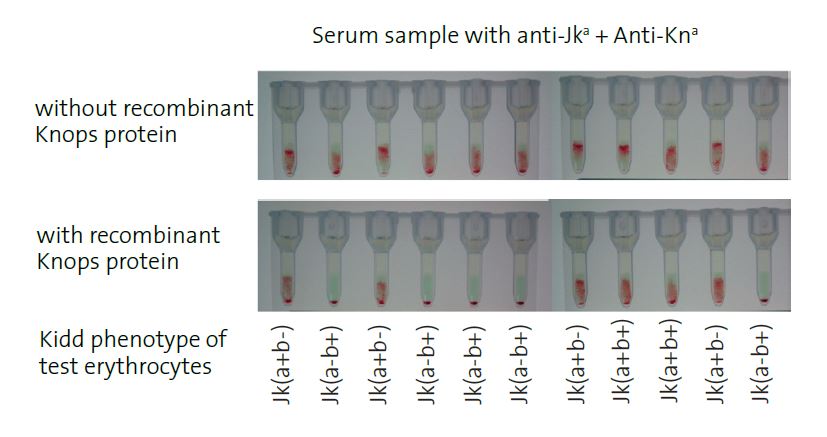
18 specific kits available: k, Lu (b), Yt (a), JMH … Example. Specific detection of alloantibodies mixed in the presence of anti-Kn (a):